When water is confined at high pressure between sheets of graphene its molecules adopt a square configuration, says a team of physicists from the University of Science and Technology of China, the University of Manchester, UK, and the University of Ulm, Germany.
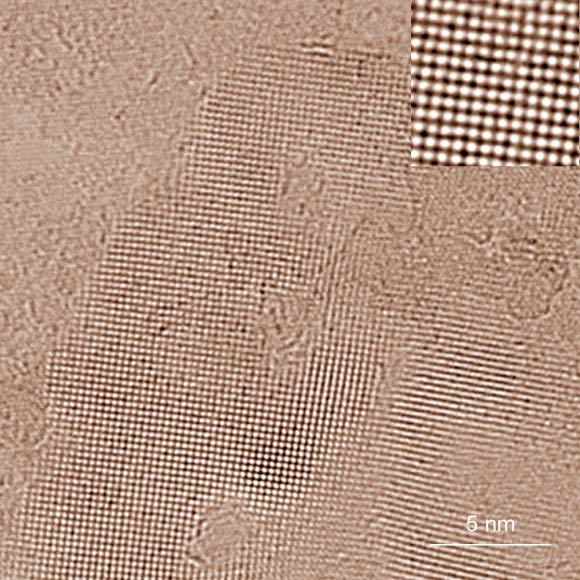
Square ice between two graphene sheets as seen in a transmission electron microscope (TEM); high-contrast dark spots are oxygen atoms that indicate positions of water molecules; hydrogen atoms yield too little contrast to be resolved even by the TEM. The top right inset shows a magnified image of a small area in the centre of the ice crystal. Image credit: University of Ulm.
In our everyday lives we are familiar with water in its more common liquid, ice and vapor forms.
Researchers also study water under more extreme conditions, including at high pressures, where it can exist in the solid state even at room temperature.
Ice crystals form in the beautifully symmetric tetrahedral shapes seen in snowflakes and on the surface of frozen ponds. Such geometries can persist at very high pressures, even if the underlying structure undergoes phase changes both subtle and dramatic with varying pressure. This certainly applies to unconstrained water.
When confined between other materials, however, the behavior of water is influenced by atomic interactions with material surfaces.
In a new study carried out by Dr Gerardo Algara-Siller of the University of Ulm and his colleagues, a graphene monolayer was first deposited on an electron microscope grid, and then exposed to a droplet of water and covered with another layer of graphene.
Much of the water was squeezed out of the graphene sandwich by the van der Waals force. The remainder was trapped in pockets less than a millionth of a meter across.
“We did not know at first what we were seeing, and only in discussion with our Manchester colleagues was the idea of square ice born,” said Prof Ute Kaiser of the University of Ulm, a co-author on the study.
“A detailed structural and elemental analysis then proved this structure to be real.”
The results, reported in the journal Nature, could improve our understanding of water transport through nanometer-scale channels in natural and artificial membranes.
http://www.sci-news.com/physics/science-new-form-square-ice-02637.html