金腾川团队揭示B型链球菌成孔蛋白CAMP因子的分子机制
中国科学技术大学生命科学学院金腾川课题组和加拿大滑铁卢大学Michael Palmer课题组合作,利用X晶体衍射技术首次解析B型链球菌成孔蛋白CAMP因子的晶体结构,并揭示了该毒力因子行使生物学功能的分子机制。研究成果以“Crystal structure of the Streptococcus agalactiae CAMP factor provides insights into its membrane-permeabilizing activity”为题,于6月8日在线发表在Journal of Biological Chemistry杂志。
B型链球菌是导致孕期/围产期胎儿严重感染性疾病的主要病原菌,其中CAMP因子是B型链球菌所分泌的毒力因子(溶血素),它通过跨膜形成孔径而造成细胞裂解(CAMP反应)。CAMP反应已经发现70多年并曾经广泛用于临床检测B型链球菌感染,但CAMP因子跨膜形成孔径的结构生物学机理仍不清楚。本研究通过解析CAMP因子的晶体结构,发现其具有全新的蛋白折叠。进一步确定其N端和C端两个不同区域的独特功能,初步阐述其形成跨膜孔径的分子机理,从而在分子层次解释CAMP反应这一现象。本项目对筛选抗CAMP毒力因子的药物有一定理论指导意义,同时对揭示蛋白与生物膜相互作用的本质有着很重要的启示作用。
该工作由金腾川课题组和加拿大滑铁卢大学Michael Palmer课题组共同合作完成。第一作者为金腾川老师和Eric Brefo-Mensah博士,金腾川老师为通讯作者。
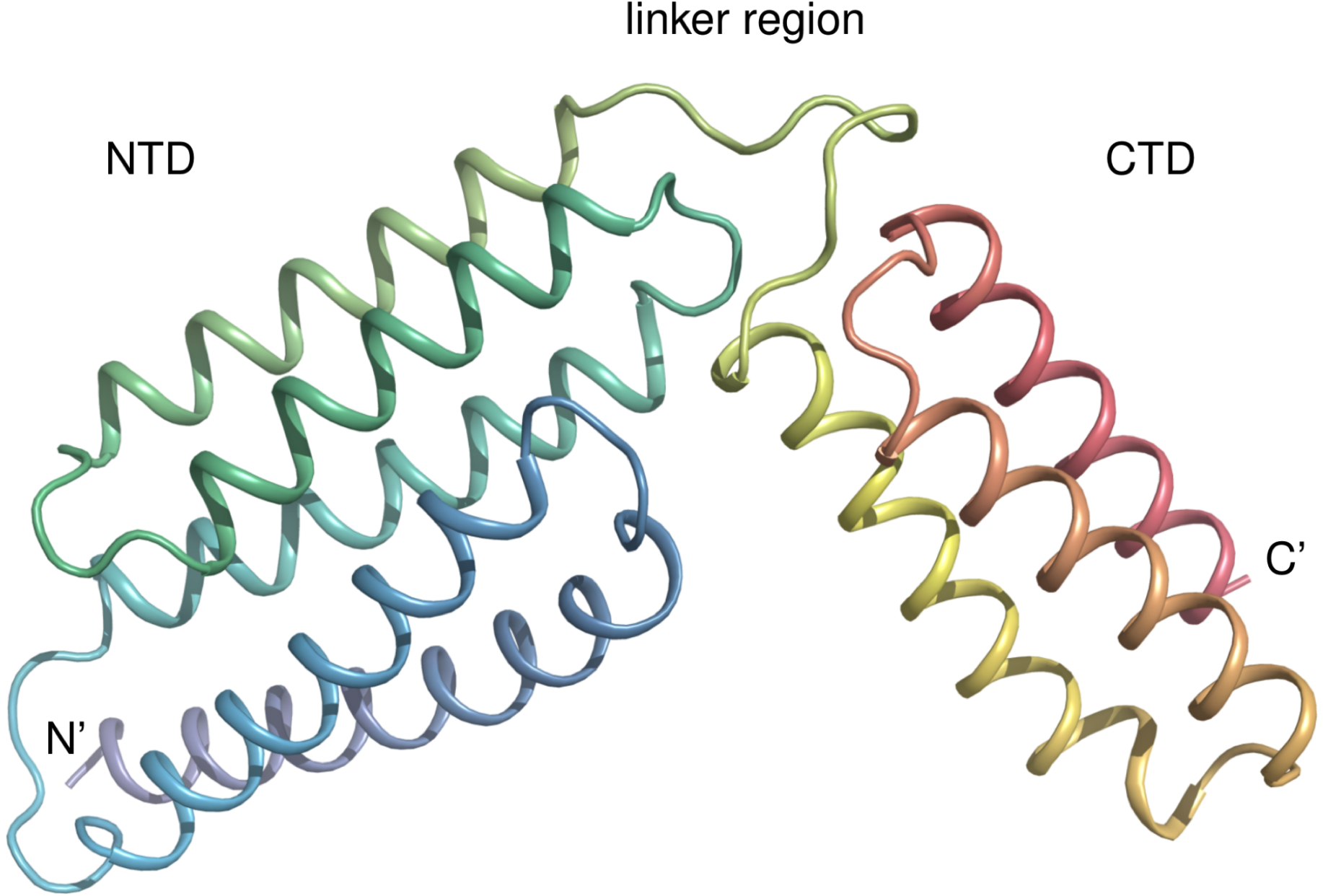
B型链球菌毒力因子CAMP因子的晶体结构
论文链接: http://www.jbc.org/content/early/2018/06/08/jbc.RA118.002336.long
ABSTRACT
Streptococcus agalactiae is an important human opportunistic pathogen that can cause serious health problems, particularly among newborns and older individuals. S. agalactiae contains the CAMP factor, a pore-forming toxin first identified in this bacterium. The CAMP reaction is based on the co-hemolytic activity of the CAMP factor and is commonly used to identify S. agalactiae in the clinic. Closely related proteins are present also in other Gram-positive pathogens. Although the CAMP toxin has been discovered more than a half century ago, no structure from this toxin family has been reported, and the mechanism of action of this toxin remains unclear. Here, we report the first structure of this toxin family, revealing a structural fold composed of 5+3 helix bundles. Further analysis by protein truncation and site-directed mutagenesis indicated that the N-terminal 5 helix bundle is responsible for membrane permeabilization, whereas the C-terminal 3 helix bundle is likely responsible for host receptor binding. Interestingly, the C-terminal domain inhibited the activity of both full-length toxin and its N-terminal domain. Moreover, we observed that the linker region is highly conserved and has a conserved DLxxxDxAT sequence motif. Structurally, this linker region extensively interacted with both terminal CAMP factor domains, and mutagenesis disclosed that the conserved sequence motif is required for CAMP factor’s co-hemolytic activity. In conclusion, our results reveal a unique structure of this bacterial toxin and help clarify the molecular mechanism of its co-hemolytic activity.
(生命科学学院 科研部)